|
Research programs Research
Interests Techniques Representative Projects FAQ
Representative projects of ligand-protein
interactions:
- Development of calmodulin inhibitors from
screening studies
Calmodulin
(CaM) (calcium-modulated
protein) is a small (148 amino acids long, 16.7 Kda)
multifunctional calcium-binding protein expressed in many cell types.
Binding calcium ions (Ca2+) is required for its activation, and
up to four calcium ions are bound by calmodulin. Once bound to calcium
ions, calmodulin acts as part of calcium signal transduction pathway, and
subsequently it mediates many crucial processes by binding various targets
(e.g. enzymes, other proteins) in the cell. Many of these targets, that
calmodulin binds, are unable to bind calcium themselves, and use calmodulin
as a calcium sensor and signal transducer. This function of calmodulin is
very important, because it indirectly plays a role in many physiological
processes. Inhibiting or modulating the actions of calmodulin is thus
essential in controlling different biological processes and diseases.
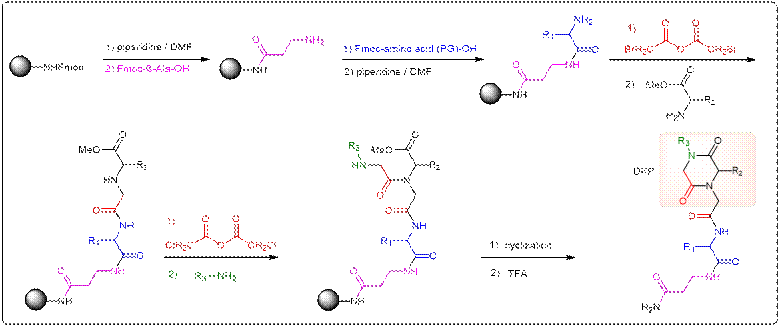
In this
project, libraries of small synthetic compounds (e.g., diketopiperazines
(DKPs), figure below) are prepared by solid-phase synthesis on a special
type of cellulose membrane using combinatorial synthesis and technologies
(e.g. SPOT synthesis). Such cellulose-bound compounds may be tested in situ
for protein binding (macroarray), or can be
dissolved to give cellulose-compound conjugates that can be transferred to
the surface of a special glass carrier to produce an array (mini- or a
microarray) of several thousands of analogues, which can then be used for
the screening of selective ligands to a set of proteins.
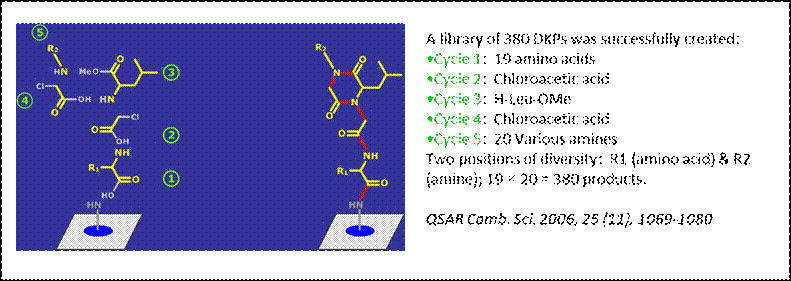
Screening
experiments of these DKP arrays with calmodulin yielded active structures.
These positive “hits” were synthesized (scheme below) and purified for
subsequent in vitro and in vivo studies.
- Development of lipopeptides bearing
photoreactive groups for investigating the mechanisms of activation of
toll-like receptors
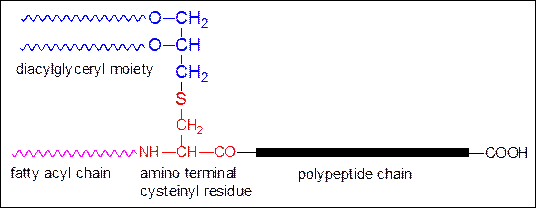
TLRs sense
bacteria and initiate an innate immune response. TLR2 transmits signals
into the cell in response to bacterial lipoproteines
(BLP) (Fig.) which are expressed by all prokaryotes. The characteristic
amino terminal lapidated cysteine is necessary for TLR activation.
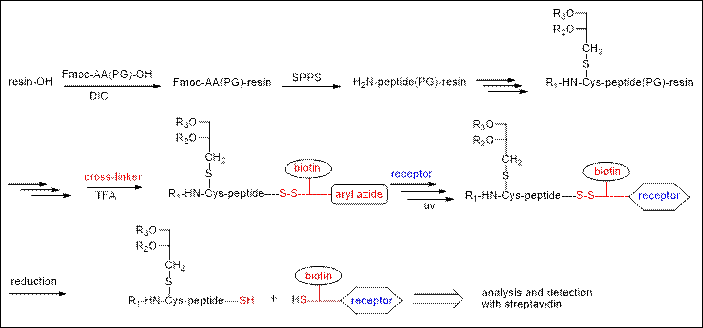
The work
aimed to find a suitable substrate to study a protein-ligand interaction. I
have designed and performed the synthesis and the purification of
lipopeptides as analogue of BLP. Peptides were prepared by applying the Fmoc-based solid-phase peptide synthesis. The Synthesis
was performed manually and on a fully automatic peptide synthesizer
(Applied Biosystems ABI 433A Peptide Synthesizer), while peptide
collections were synthesized on automated simultaneous multiple peptide
synthesizer (Advanced ChemTech 348 MPS and MultiSynTech Syro2000). The automated synthesis was
achieved via double coupling programs. Side-chain modifications have been
managed getting use of orthogonal protecting groups. Bacterial lipopeptides
were then successfully synthesized on the solid support by elongation of
the peptides with Fmoc-Cys(2,3-dihydroxypropyl)-OH,
followed by the necessary reactions (O-acylation, Fmoc-deprotection, N-acylation, …). The
resin-bound lipopeptides were labeled with a
suitable cleavable trifunctional cross-linker and with a radiolabelled
fatty acid. After their release from the solid support, the compounds were
purified by HPLC (JASCO, Shimadzu), and their structures were characterized
by MALDI-MS. The labelled lipopeptides were allowed to bind to their
receptor (protein-ligand interaction) with subsequent photoactivation and cleavage
of the disulfide link (label transfer).
Although
molecular mechanisms enabling the detection of lipopeptides by TLR2 are
unknown, we could show the serum protein vitronectin and its receptor integrin
β3 recognize lipopeptides and contribute to the initiation of TLR2
responses. Our findings indicate that integrin β3 is
important during microbe-induced inflammation and can provide a new target
for clinical intervention (Nature Immunology 2008).
|